In chemistry, glass is a solid material primarily composed of silicon dioxide (SiO2) with varying amounts of other oxides, such as sodium oxide (Na2O), potassium oxide (K2O), calcium oxide (CaO), and aluminum oxide (Al2O3). The term “glass” refers to an amorphous or non-crystalline solid, which means that its atomic structure lacks long-range order like regular crystals. Instead, the atoms in glass are arranged in a disordered and random fashion, giving it unique properties compared to crystalline solids.
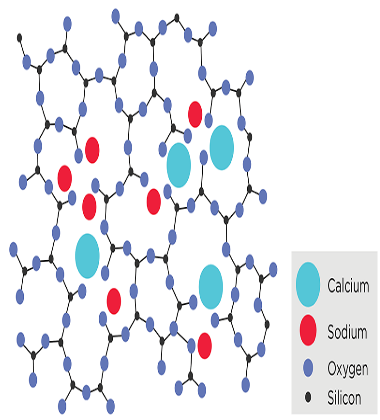
Composition and Types of Glass: The primary component of glass is silica (SiO2), which is abundant in nature as quartz or sand. When heated to high temperatures, silica melts and can be shaped into various forms, but upon cooling, it solidifies without forming a regular crystal lattice, leading to the formation of glass.
The specific properties of glass can be fine-tuned by adding other oxides to the silica. For instance, the addition of sodium oxide lowers the melting point, making it easier to shape the glass at lower temperatures, resulting in what is known as soda-lime glass, commonly used in household glassware. The addition of lead oxide leads to lead glass or lead crystal, which has high refractive index and excellent optical properties, often used in fine glassware and decorative items. Borosilicate glass is made by adding boron oxide to silica, giving it exceptional thermal shock resistance and making it suitable for laboratory glassware and heat-resistant cookware.
Formation of Glass: The formation of glass involves the process of vitrification, which occurs when a suitable mixture of raw materials is heated to high temperatures and then rapidly cooled (quenched) to prevent the formation of a crystalline structure. This rapid cooling traps the atoms in a disordered arrangement, creating the amorphous solid known as glass.
Properties of Glass: Glass possesses several important properties that make it widely useful in various applications:
- Transparency: Most glasses are transparent to visible light, allowing light to pass through with minimal absorption or scattering. This property is essential for glass used in windows, lenses, and optical devices.
- Brittleness: Glass is a brittle material, meaning it is prone to cracking or shattering when subjected to mechanical stress. This property should be considered when designing and using glass objects.
- Chemical Inertness: Glass is chemically inert and does not react with most substances, making it suitable for storing and transporting chemicals in laboratories and industries.
- Thermal Stability: Different types of glass have varying degrees of thermal stability. Some glasses can withstand rapid changes in temperature without breaking, while others are more susceptible to thermal shock.
- Electrical Insulator: Glass is an excellent electrical insulator, making it suitable for electrical and electronic applications.
- Recyclability: Glass is highly recyclable, and it can be melted down and reshaped multiple times without a significant loss in quality, making it an environmentally friendly material.
Conclusion:
In chemistry, glass refers to an amorphous solid primarily composed of silica (SiO2) and other oxides. Its unique atomic structure without long-range order gives glass distinct properties, including transparency, brittleness, chemical inertness, and thermal stability. Glass has a wide range of applications in everyday life, scientific research, industries, and technology, making it an indispensable material in modern society.


